01. Mesothelioma Immunotherapy
What Is Mesothelioma Immunotherapy?
Mesothelioma immunotherapy uses a patient’s immune system to find and attack cancer cells. One type, called immune checkpoint inhibitors, is a standard treatment for mesothelioma. But researchers have explored many types of immunotherapy, including:
- Cancer vaccines
- CAR T-cell therapy
- Immune checkpoint inhibitors
- Monoclonal antibodies
- Oncolytic viruses
Checkpoint inhibitors have made important strides. These new drugs have extended survival and improved quality of life for some mesothelioma patients. Checkpoint inhibitors also tend to be less toxic than chemotherapy drugs.
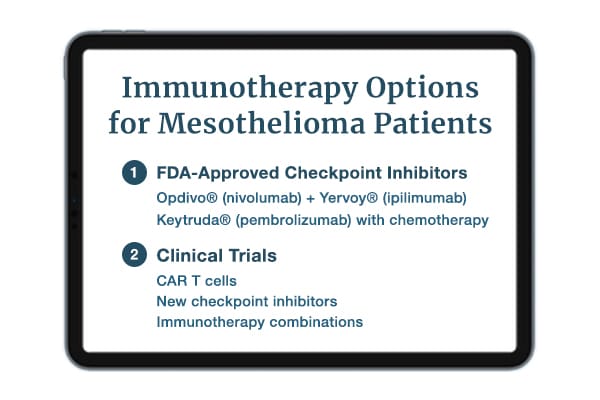
FDA-Approved Mesothelioma Immunotherapy
The United States Food and Drug Administration (FDA) has approved some immunotherapy drugs for mesothelioma. Others have FDA approval to treat tumors with characteristics found in some mesotheliomas. FDA-approved immunotherapies for mesothelioma include:
- Opdivo® + Yervoy®: Opdivo (nivolumab) has FDA approval to treat inoperable malignant pleural mesothelioma when combined with Yervoy (ipilimumab). In one pleural mesothelioma study, this combination improved overall survival versus chemotherapy. Study patients treated with the combination had a median survival of 18.1 months.
- Keytruda®: Keytruda (pembrolizumab) is also approved for inoperable malignant pleural mesothelioma when combined with pemetrexed and platinum chemotherapy. Keytruda on its own is approved to treat tumors in the “tumor mutational burden-high” (TMB-H) category. If a patient’s mesothelioma tumor falls into this category, doctors can prescribe Keytruda as a treatment. In one study, the median overall survival was 18 months with Keytruda.
Clinical trials continue to investigate immunotherapy drugs for treating mesothelioma. Interested patients should discuss immunotherapy with their oncologists.
Mesothelioma Immunotherapy & Multimodal Treatments
Some doctors are now crafting multimodal treatments that include immunotherapy. Multimodal treatments combine two or more therapies into a single plan. This allows patients to benefit from more than one cancer-fighting treatment at once.
Doctors and researchers have already begun looking into using immunotherapy before or after chemo. And some small studies have reported encouraging results from other immunotherapy combinations.
One study treated pleural mesothelioma patients with Tecentriq® (atezolizumab) and chemo. Then patients had surgery. They also had the option of receiving radiation or additional Tecentriq for up to a year. Interim results suggest the median survival will be better than 20 months.
One of the study authors described the success of this approach as “rather encouraging.” In the future, researchers may find ways to extend survival even further with immunotherapy combinations.
Who Is Eligible for Immunotherapy?
There are two FDA-approved immunotherapy treatments for mesothelioma. In general, mesothelioma patients who do not qualify for surgery may be eligible. This means many patients may qualify for these forms of immunotherapy. But eligibility can also depend on other factors.
Interested patients should discuss immunotherapy with a qualified specialist. Experienced mesothelioma doctors can discuss eligibility and help patients weigh the benefits and risks.
02. How It Works
How Does Immunotherapy for Mesothelioma Work?
Immunotherapy works by allowing the immune system to recognize and attack cancer cells. Each type of immunotherapy takes a slightly different approach to this. CAR-T cell therapy reprograms immune cells to make them attack tumors.
Checkpoint inhibitors block cancer cells from using immune checkpoints. These checkpoints normally allow immune cells to recognize and ignore healthy cells. But cancer cells can use checkpoints to pose as healthy and dodge immune cell attacks. Checkpoint inhibitors take this option out of play, allowing the immune system to fight tumors.
On their own, checkpoint inhibitors may improve life expectancy and quality of life. For some patients, checkpoint inhibitors may also provide effective palliation. Palliative care aims to relieve cancer symptoms and improve quality of life. Some data suggests these checkpoint inhibitors may be even more helpful when combined with other treatments.
Resources for Mesothelioma Patients
“I appreciate how easy your guide has made it to read and learn about this cancer. I am beyond grateful for all Mesothelioma.com has done to help.”
A Mesothelioma Patient
03. Benefits of Immunotherapy
Immunotherapy Benefits for Mesothelioma
Immunotherapy offers several potential benefits for mesothelioma, including better prognosis and quality of life. Benefits vary depending on the type of immunotherapy, the tumor location and patient factors.
In studies, researchers have observed immunotherapy benefits in several areas:
- Preparation: Some immunotherapy drugs do not require pre-treatment regimens the way certain chemotherapy drugs do. For example, patients receiving pemetrexed chemotherapy get vitamin injections the week before treatment. Checkpoint inhibitors do not require this preparation.
- Quality of life: Patients receiving immunotherapy may see quality-of-life benefits. In studies, patients receiving Opdivo and Yervoy experienced improved quality of life.
- Side effects: Patients may find immunotherapy side effects more tolerable than those of chemo. Common immunotherapy side effects include fatigue, muscle aches, fever, chills, dizziness and weakness. These may be less bothersome than the nerve pain and hearing damage sometimes caused by chemo.
- Survival: Some immunotherapies have improved survival rates versus other treatments. In one study, patients treated with Opdivo + Yervoy had a one-year survival rate of 68%. Study patients treated with chemotherapy had a one-year survival rate of 58%.
To learn more about potential immunotherapy benefits, mesothelioma patients should contact their doctor. Mesothelioma doctors can further explain the benefits relating to a patient’s individual case.
What Is the Success Rate of Immunotherapy for Mesothelioma?
The CheckMate 743 trial followed pleural mesothelioma patients treated with Opdivo + Yervoy. It reported several successes. The median survival was 18.1 months, and the 2-year survival rate was 41%. About 16% of patients didn’t see evidence of their cancer spreading for two years or more.
Patients should discuss treatment expectations for any mesothelioma therapy with an experienced oncologist. The doctor can discuss immunotherapy study results and what they may mean for the patient.
Is Immunotherapy Better Than Chemotherapy?
Doctors may consider checkpoint inhibitors superior to chemotherapy in some cases. Checkpoint inhibitors may cause more manageable side effects. In a pleural mesothelioma study, they improved survival and quality of life compared with chemotherapy.
This may not hold true for other immunotherapies. In such cases, chemotherapy may be right for some patients. People considering these therapies can discuss them with their doctors. An oncologist can help weigh the benefits and risks for each patient’s unique situation.
04. Side Effects of Immunotherapy
Mesothelioma Immunotherapy Side Effects
Immunotherapies can cause side effects when overactive immune cells attack some healthy tissues. Common reactions include fatigue, body aches and pains, rashes and diarrhea.
Immunotherapy side effects can range from mild to severe depending on many factors. These include the type of immunotherapy, tumor location and individual patient factors. For checkpoint inhibitors, experts say most side effects are manageable with standard approaches. Patients can discuss possible side effects with their doctors before treatment.
Mesothelioma Immunotherapy Side Effects in Real Life
Doctors tracked side effects reported by mesothelioma patients in the Opdivo + Yervoy clinical trial. More than 30% of study patients experienced:
- Fatigue
- Muscle and body pain
- Skin rash
- Diarrhea
Some patients experienced serious side effects like pneumonia and other respiratory problems. Most serious reactions resolved with steroids or other supportive treatments.
Even if they seem manageable, immunotherapy side effects should be promptly reported to healthcare providers. This can help doctors and nurses respond if a reaction becomes severe.
05. Types of Immunotherapy
Types of Immunotherapy Treatments
Immunotherapies turn the immune system against cancer, but they use a variety of tactics to do so. Checkpoint inhibitors like Opdivo and Yervoy block a loophole that cancer uses to hide from immune cells. And monoclonal antibodies can mark cancer cells for destruction.
Some immunotherapies already have FDA approval for mesothelioma. Others are under investigation for lung cancer, mesothelioma and other cancers. Immunotherapies investigated or approved for mesothelioma include:
- Cancer vaccines
- CAR T cells
- Immune checkpoint inhibitors
- Monoclonal antibodies
Each type of immunotherapy comes with its own potential benefits and risks.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors are a type of monoclonal antibody. They allow the immune system to recognize and attack cancer cells. The checkpoint inhibitor duo of Opdivo + Yervoy has FDA approval for treating inoperable pleural mesothelioma.
Checkpoint inhibitors block specific loopholes (immune checkpoints) that cancer cells use to hide from the immune system. For mesothelioma, several of these drugs target the PD-1/PD-L1 checkpoint, including Imfinzi® (durvalumab). Others — like Imjudo® (tremelimumab) — target the CTLA-4 checkpoint. Blocking these checkpoints helps the immune system recognize and kill cancer cells.
The tactic of immune checkpoint blockade has worked well in mesothelioma studies. In a clinical trial, Opdivo + Yervoy achieved a median survival of 18.1 months. Study patients also experienced improved quality of life with Opdivo + Yervoy versus chemotherapy. Other checkpoint inhibitors are the subject of ongoing clinical trials.
Monoclonal Antibodies
Monoclonal antibodies (mAbs) are antibodies that help the immune system identify dangerous materials or cells in the body. In cancer treatment, mAbs can be used to let immune cells identify and fight tumors.
So far, the FDA has approved a few monoclonal antibody treatments for cancer. Approved mAb treatments for mesothelioma include immune checkpoint inhibitors, a type of monoclonal antibody. As more clinical trials are completed, additional drugs may be approved.
CAR T-Cell Therapy
CAR T-cell therapy uses modified immune cells to fight cancer. Normal T cells, also known as white blood cells, protect the body from disease. But cancer cells know how to trick normal T cells into overlooking them. CAR T-cell therapies fix this by reprogramming T cells to recognize and fight cancer.
A recent study used CAR T cells to treat pleural mesothelioma. Patients had undergone standard treatment before enrolling. In the study, they received CAR T cells and Keytruda® (pembrolizumab). This treatment achieved a median survival of 23.9 months. This represents an improvement of more than a year compared to other second-line treatments.
As of 2022, the FDA has not approved CAR T-cell therapy for treating mesothelioma. This technology does have FDA approval to treat some other forms of cancer. CAR T-cell therapies are an active area of mesothelioma research.
Cancer Vaccines
Cancer vaccines are a form of immunotherapy. They aim to prevent or treat cancer by teaching the immune system to see cancer or its precursor as an intruder. This allows immune cells to attack the cancer cells. Some cancer vaccines may allow the body to keep fighting cancer long after the patient is vaccinated.
Mesothelioma cancer vaccines show immune cells how to recognize malignant mesothelioma tumors. These vaccines have shown some promise in treating pleural mesothelioma patients.
06. Future of Immunotherapy
The Future of Immunotherapy for Mesothelioma
Experts say the future of mesothelioma immunotherapy will feature chemoimmunotherapy. Researchers may also combine immunotherapy with surgery or radiation. But chemoimmunotherapy has already been tested in a few small studies. One reported an encouraging median survival of 20.4 months.
Future studies may also explore how immunotherapy can impact eligibility for surgery. In the past, many patients were not considered for surgery because of their cell type. Some mesothelioma cell types have a reputation for responding poorly to treatment.
But immunotherapy is changing that reputation. A recent report published evidence of Opdivo + Yervoy making a patient with a difficult cell type eligible for surgery. Additional studies may help this become a more regular occurrence.
Recent and Upcoming Clinical Trials
07. Common Questions
Common Questions About Mesothelioma Immunotherapy
-
Can immunotherapy be used for mesothelioma?
Immunotherapy has recently become a standard treatment for mesothelioma. The Opdivo + Yervoy combo is FDA-approved for inoperable pleural mesothelioma. Data indicates this combo can extend survival and improve quality of life. Patients interested in mesothelioma immunotherapy should discuss it with a specialist.
-
Can immunotherapy cure mesothelioma?
No. Immunotherapy can’t cure mesothelioma, but it can extend survival and improve quality of life.
Answered by Vincent Mase, M.D. -
How long does immunotherapy work for mesothelioma?
In studies, some patients have experienced immunotherapy benefits lasting for years. Others have not. In the CheckMate 743 trial, some patients saw Opdivo + Yervoy hold tumors at bay for up to two years. But knowledge of immunotherapy benefits for mesothelioma is still evolving. Interested patients should discuss this topic with a mesothelioma specialist. The doctor can explain study data and share their professional experiences.
- What are the best immunotherapy treatments for mesothelioma?
-
How much does immunotherapy cost?
The combo of Opdivo + Yervoy is the only approved immunotherapy for mesothelioma. According to the manufacturer, the cost for a single round of Opdivo + Yervoy for pleural mesothelioma is $27,194. Some insurance plans, including Medicare, may cover all or part of this cost.